Update: April 23rd 2020:
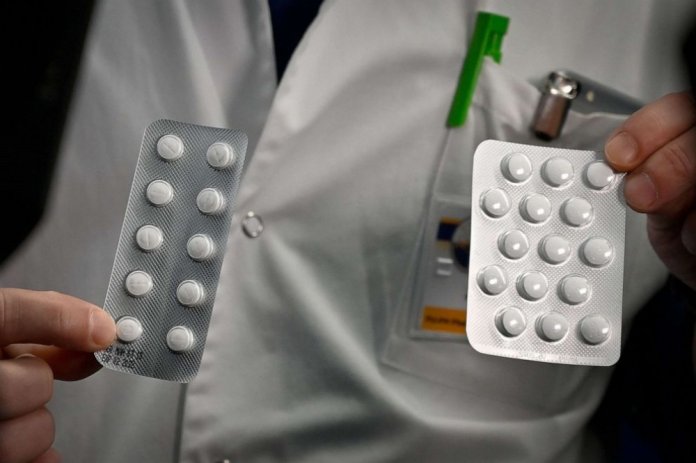
According to a recently published study investigating the outcomes of coronavirus patients treated with hydroxychloroquine, it concluded that the medicine is ineffective in treating COVID-19 patients.
Here’s how the research was conducted
The research titled “Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19,” observed 368 coronavirus patients after they had been treated by antiviral drug along with or without azithromycin. Researchers have claimed that this is by far the most comprehensive study conducted to ascertain the power of hydroxychloroquine.
The study was carried out by involving 368 patients, it’s the largest look so far of hydroxychloroquine with or without the antibiotic azithromycin for COVID-19, which has killed more than 171,000 people as of Tuesday.
Moreover, the study concluded that 28% of patients treated with only hydroxychloroquine died while 22% of patients treated with hydroxychloroquine, along with azithromycin, died as well. Whereas, only 11% of the patients who received routine care died.
They also found that the organs of the patients who died after receiving hydroxychloroquine with or without azithromycin were severely damaged in comparison to patients who died while receiving normal care.
The drug Hydroxychloroquine is known for its adverse side-effects. These side-effects include nausea, stomach cramps, headache, loss of appetite, diarrhea, dizziness, and blurred vision. Also, in extreme conditions, the antiviral drug can cause fast or irregular heartbeat, leading to death.
The ongoing pandemic that is caused by coronavirus also known as COVID-19 has caused chaos globally. Various studies and researches are being carried out in order to make a vaccine that could provide a cure to the deadly virus.
Recently, U.S President Donald Trump claimed during a White House briefing on Thursday that the Food and Drug Administration (FDA) had approved a ‘very powerful’ drug known as chloroquine to treat COVID-19. Chloroquine is a drug commonly used to treat malaria, lupus and rheumatoid arthritis.
Pres. Trump touts chloroquine, an old malaria drug, that doctors say may help treat novel coronavirus, claims it will be available “almost immediately.”
Read more about chloroquine: https://t.co/r20TMjfgaQ pic.twitter.com/3OSBmjCC8e
— ABC News Politics (@ABCPolitics) March 19, 2020
This news shared by President Trump was met with mixed reactions, while some were ecstatic and it provided hope to many, others were confused as to why this information was not known to anyone and whether this was actually true.
Is Chloroquine FDA Approved for COVID-19?
However, the drug has not yet been approved by the FDA as Trump claims. FDA commissioner, Steven Hahn has clarified that the agency is still carrying out trials on different drugs to determine if they can be used for treating COVID-19.
Hahn further mentioned that chloroquine is approved for use against malaria and in some cases arthritis however it is not yet approved to treat the novel coronavirus. Clinical trials are still being held to determine the impact of the drug on a patient battling covid-19.
Steven Hahn also added in a statement,
“we also must ensure these products are effective; otherwise we risk treating patients with a product that might not work when they could have pursued other, more appropriate, treatments.”
Initial trials have shown positive results when chloroquine was used to treat COVID-19 but still, these were not deemed enough to officially gain FDA approval.
President Trump also spoke about another Anti-Viral drug called, Remdesivir, saying it “has been approved or very close to approved” by the FDA.
Remdesivir has been allowed by the FDA to be used in clinical trials agains Coronavirus and to be used under a compassionate use rule. This rule means doctors can use new or unapproved drugs in cases where there are no treatment options. However the FDA has clarified again that it has not approved any drugs to threat COVID-19 yet.
Presently there are no FDA-approved products to prevent, treat or cure #COVID19. However, bad actors are hawking products online and in stores that claim to do just that. Be wary of these scams and talk to your doctor before using any such product.
— Dr. Stephen M. Hahn (@SteveFDA) March 17, 2020
The pharmaceutical company, Bayer announced on Thursday that it is donating 3 million tablets of its chloroquine phosphate drug, sold under the name Resochin, to the US government for trials.
After the announcement made by Trump, medicines containing chloroquine have started disappearing from markets globally. Pakistani stores have also experienced the same this morning!
Chloroquine Demand Increases in Pakistan!
Suddenly, #Chloroquine tablet Resochin, has disappeared from markets in Karachi. #CoronaVirusPakistan pic.twitter.com/xLyCESLBNv
— سرفراز احمد خان (@sa_cubes) March 20, 2020
Chloroquine has also been used for the treatment of COVID-19 cases in France, China and South Korea. They have labeled the drug as an effective antiviral treatment against the disease.
Follow Brandsynario for the latest news and updates.